ABOVE: MicroRNAs, small noncoding molecules, play an important role in posttranscriptional gene regulation and offer new avenues for understanding complex diseases. ©iStock, Artur Plawgo
Victor Ambros and Gary Ruvkun were awarded the 2024 Nobel Prize in Physiology or Medicine for their groundbreaking discovery of microRNA and its role in post-transcriptional gene regulation. Their journey began in the late 1980s while studying the development of the Caenorhabditis elegans worm under the mentorship of Robert Horvitz. Through their investigations, they identified the lin-4 gene, which did not code for a protein but instead produced short RNA transcripts—later dubbed microRNAs—that regulated the lin-14 gene, showcasing a new principle of gene regulation.1 Although initially considered an anomaly in worms, their work paved the way for the discovery of hundreds of microRNAs encoded in the human genome, many of which are conserved in other animals.
Their discovery, 30 years on, has helped advance scientists’ understanding of embryological development and diseases like cancer, illustrating how research on simple model organisms can yield insights that are relevant to human health. Although scientists continue to highlight their importance in health and disease, making these mini molecules into medicines has proven challenging.
Continue reading our coverage of this year’s prize for more on the history of microRNAs.
A microRNA Cluster Shapes Sex Determination
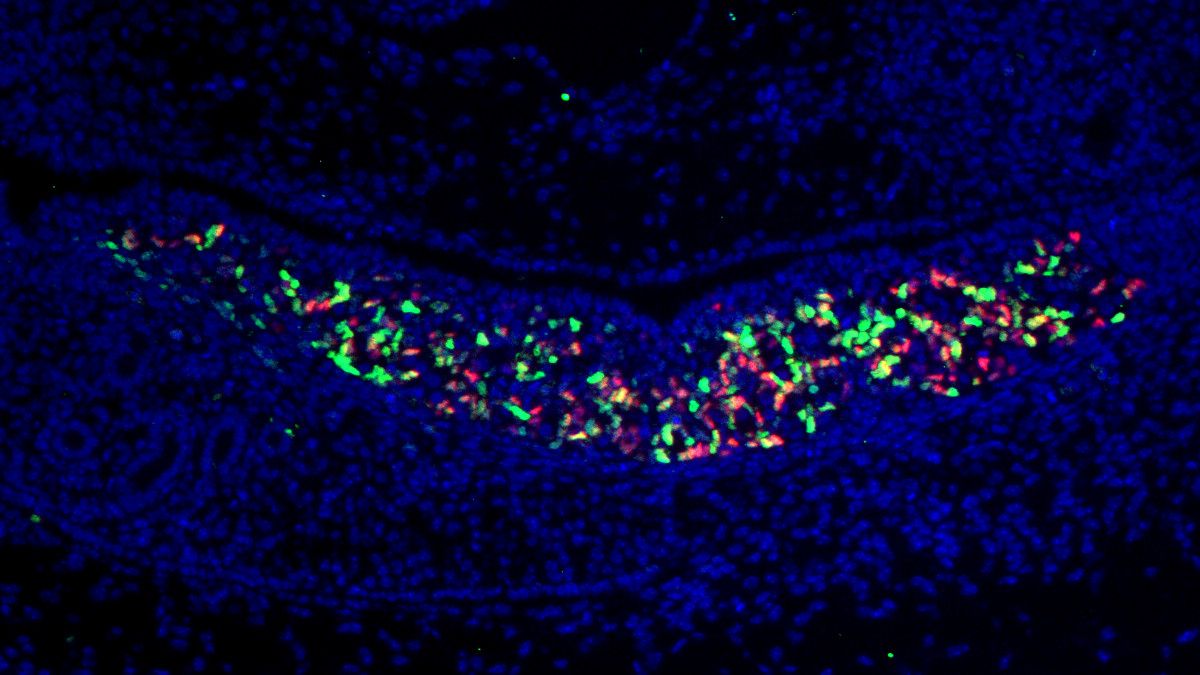
While past research on sex determination has primarily concentrated on identifying the role of different genes and the molecules they encode, the involvement of noncoding microRNAs remains largely unexamined. In a study published in Nature Communications, scientists revealed that deleting a cluster of microRNA in embryonic mice resulted in the transformation of male (XY) mice into females, demonstrating a complete sex reversal. The research team, led by geneticists Francisco Barrionuevo and Darío Lupiáñez, found that a single microRNA cluster—miR-17~92— is essential for the development of Sertoli cells, which support sperm development. Deletion of miR-17~92 caused delayed expression of Sry, crucial for initiating testis development. These findings not only highlight the importance of noncoding elements in sex determination but also suggest a conserved mechanism that could have implications for understanding sexual development disorders in humans.
Explore the role of microRNAs in sex determination further in this article.
Offsetting Anxiety with the Help of a Small RNA
Nearly one-third of adults in the US will experience an anxiety disorder during their lifetime. However, current medications are not effective for everyone. Valentina Mosienko, a neuroscientist at the University of Bristol, hopes that by understanding the molecular basis of anxiety scientists can develop better treatments. In a study published in Nature Communications, Mosienko and her team investigated the role of microRNAs in stress-induced anxiety in mice. Focusing on the amygdala, a brain region implicated in anxiety regulation, Mosienko and her team discovered that stress significantly increased the expression of miR-483-5p. The microRNA downregulated stress-related genes, notably Pgap2, in neuronal cell cultures. Injecting miR-483-5p into mice amygdalae reduced anxiety, while blocking its action on Pgap2 reversed this effect. These findings suggest that miR-483-5p helps mitigate stress and anxiety, potentially paving the way for new anxiety treatments targeting similar pathways in humans.
Learn more about the study here.
A MicroRNA Family Solves a Memory T Cell Mystery
When the immune system detects a threat, CD8+ T cells become cytotoxic, rapidly proliferating to destroy infected or cancerous cells. Once the danger is eliminated, most of these cells die, leaving behind memory T cells that ensure quicker responses to future threats. However, the formation of memory T cells remains a mystery. In a paper published in Nature Communications, researchers from the University of Massachusetts Amherst discovered that the microRNA family let-7 is crucial for the formation of memory T cells. In their study, they found that overexpression of let-7 in CD8+ T cells in mice bearing melanoma tumors slowed tumor growth and promoted memory T cell formation, while its absence impeded tumor control. The researchers attributed this effect to let-7's role in inhibiting reactive oxygen species production, which is required for the development of the memory phenotype. Their research suggests that let-7 microRNA could be a promising immunotherapy to enhance the immune system's ability to fight cancer by promoting the formation of memory T cells.
Learn more about this microRNA family here.
MicroRNA Biomarkers Offer Hope for Rapid Diagnosis and Treatment
When a newborn shows signs of respiratory distress, lethargy, or seizures after a difficult birth, doctors must act fast. If the newborn baby has neonatal encephalopathy (NE)—a serious syndrome characterized by neurological dysfunction—they need to initiate treatment immediately. However, there are few reliable biomarkers for assessing brain injury severity and predicting treatment responses. A recent study revealed that specific microRNAs in blood samples could serve as promising noninvasive biomarkers for NE diagnosis and prediction. A team of researchers found elevated levels of miR-34c-5p, miR-491-5p, and miR-346 in infants with NE and an upregulation of miR-15b-5p and miR-16-5p in patients with milder injuries. The identification of miRNA biomarkers could pave the way for better, and faster, diagnostics and more targeted therapies, offering hope for improved long-term outcomes in neonates that develop NE.
Continue reading about microRNA biomarkers in this story.
MicroRNA-Based Therapeutics are Still in Their Infancy
The discovery of microRNAs not only revealed a new form of gene regulation, but it opened the door to novel approaches for regulating gene activity. MicroRNAs hold significant potential as therapeutic targets and agents due to their ability to regulate gene expression and influence various biological processes. However, no microRNA-based drugs have been approved by the Food and Drug Administration (FDA) and few have reached clinical trials. Several challenges have hindered their advancement to the clinic.2 A single microRNA can regulate hundreds of genes, making it difficult to achieve precise target specificity and predict every potential off-target effect. Several promising candidates went on to cause immune-related toxicity and, in some cases, death. Furthermore, scientists have struggled to develop safe and effective delivery systems for miRNA drugs. Despite these setbacks, scientists are hopeful that ongoing advancements in drug delivery and development will transform these mini molecules into effective medicines.
- Bartel DP. Metazoan microRNAs. Cell. 2018;173(1):20-51.
- Kim T, Croce CM. MicroRNA: Trends in clinical trials of cancer diagnosis and therapy strategies. Exp Mol Med. 2023;55(7):1314-1321.


