What’s the Difference Between a Voltage Clamp and a Current Clamp?
Depending on the “clamped” parameter, patch clamp configurations probe different aspects of a cell's electrical activity.
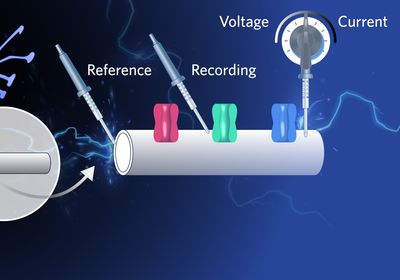
Historically, researchers impaled giant squid, snail, and frog axons with electrodes to study nerve function by measuring ion channels. Today, electrophysiologists have enhanced their ability to assess electrical impulses by clamping onto the mammalian cell membrane, improving their understanding of how cells process and transmit electrical signals. Depending on the controlled and measured variables, the voltage and current clamps are used to tune into the different cadences of these electrical conversations.
Voltage Clamp: Scrutiny of Ion Channels
The voltage clamp technique investigates the electrical properties of ion channels.1 In this method, the experimenter “clamps” or controls the membrane potential at a desired value using a feedback amplifier. The current needed to hold the membrane at the target voltage is recorded. Building on this approach, scientists developed the patch clamp technique, which is a refined version that provides greater precision by targeting a small patch of cell membrane to study individual ion channels. These methods are used to study ion channels and their kinetics by isolating specific channel activity, such as sodium, potassium, or calcium currents, while maintaining constant membrane potential.2,3
Current Clamp: Measure of Cell Excitability
Another configuration of the patch clamp technique is the current clamp, which examines how cells respond to current inputs, including the generation of action potentials and changes in membrane potential over time. Researchers inject precise amounts of current into the cell and record the resulting voltage changes, often seen as action potentials. The fluctuations in membrane potential can indicate ion channel activities, such as depolarization from voltage-gated sodium channels opening and hyperpolarization from voltage-gated potassium channels.4 This technique offers insights into the excitability of cells, such as enabling researchers to study the effects of drugs on action potential patterns.
- Neher E, et al. Pflugers Arch. 1978;375(2):219-228.
- Lai HC, Jan LY. Nat Rev Neurosci. 2006;7:548–562.
- Hodgkin AL, Huxley AF. J Physiol. 1952;117(4):500-544.
- Perkins KL. J Neurosci Methods. 2006;154(1-2):1-18.